Publications
Photo above: A 3D printed 10×10 microarray using nanoparticle for biosensing application. Adapted from Ali et al., Advanced Materials. 2021 Feb;33(7):2006647.
Cover Features:
(Click cover image to read the article.)
This Advanced Science cover image highlights a cutting-edge, 3D-printed milker-integrated microfluidic biosensor designed for real-time monitoring of key milk ions—Fe²⁺, NO₃⁻, Ca²⁺, and HPO₄²⁻—directly during milking. The schematic showcases embedded microfluidic channels and ion-selective electrodes, with molecular illustrations of ion recognition mechanisms. Integrated into automated milking systems, the device features a miniaturized, wrinkled transducer layer offering high sensitivity (as low as 1 ppm), enabling continuous, on-farm dairy health surveillance. This smart biosensor offers a powerful tool for data-driven herd management and early detection of metabolic imbalances. Photo adapted from Ali et al., Advanced Science. 2024 Dec;11(47):2470291.
The cover image from ACS Applied Materials & Interfaces highlights an advanced handheld electrochemical biosensor designed for ultra-sensitive detection of contaminants and biomarkers in milk. The foreground shows the device displaying a concentration reading of 55 pg/mL with a test strip immersed in a flowing milk stream. In the background, a dairy farm with cows and a barn emphasizes its agricultural and food safety applications. Molecular structures within the milk and a magnified inset illustrate their interaction with a 2D nanomaterial sensing surface, like graphene. This image underscores the powerful integration of nanotechnology and biosensing for rapid, on-site milk quality and safety monitoring. Adapted from Ali et al., ACS Applied Materials & Interfaces (Vol. 16, Issue 39, October 2, 2024).
The Advanced Functional Materials cover illustrates how 3D printing is revolutionizing biomedical sensor design by enabling customization, miniaturization, and flexibility. A human hand interacts with a soft, printed biosensor, while a stylized 3D printer demonstrates precise material deposition. Gold-patterned electrodes and wearable circuits highlight the potential for compact, real-time health monitoring. The image captures the power of 3D printing to create personalized, high-performance sensing devices. Adapted from Ali et al., Advanced Functional Materials. 2022 Feb 23;32(9).
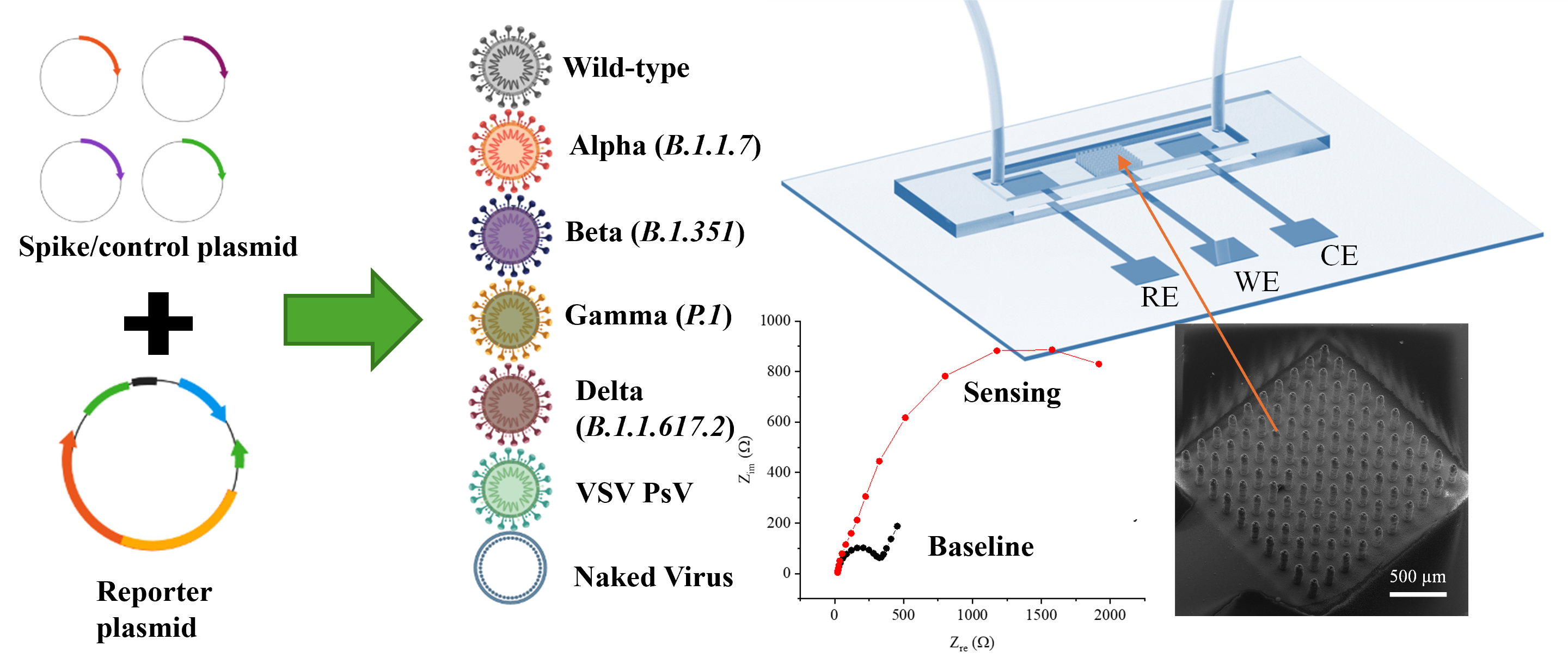
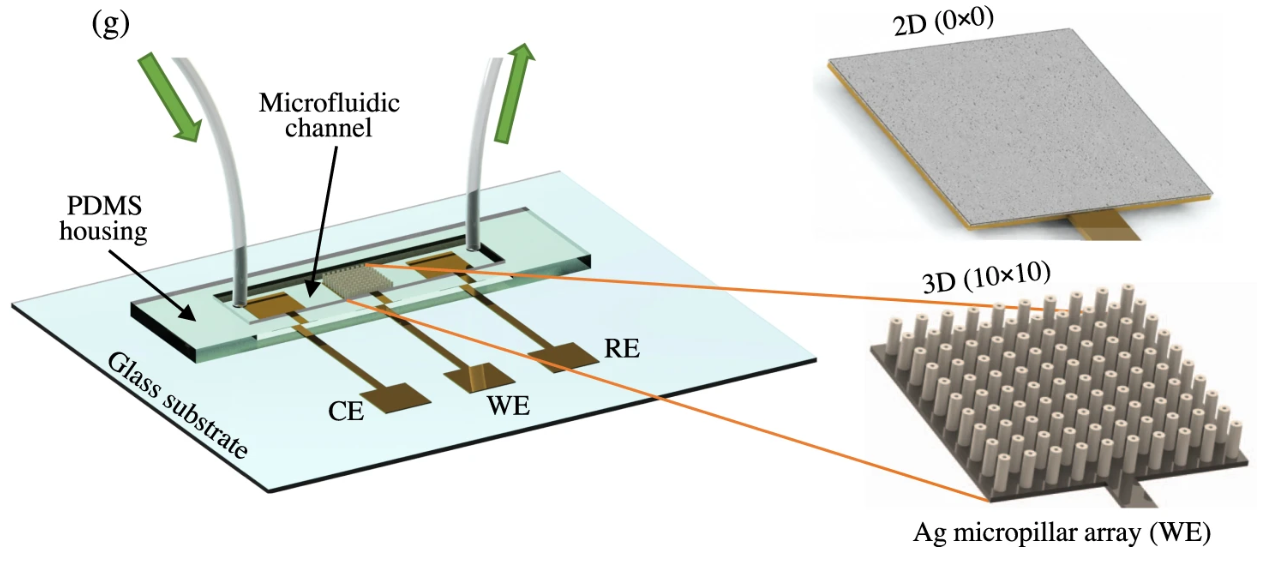
The cover image of the Journal of Medical Virology (Volume 94, Issue 12, December 2022) highlights a microfluidic electrochemical biosensor developed for detecting COVID-19 biomarkers. The illustration features a chip with integrated working, reference, and counter electrodes, where sample fluid flows across the sensing area. A scanning electron microscope (SEM) image shows a high-density 3D micropillar array on the working electrode, designed to enhance surface area and improve sensitivity. A Nyquist plot presents impedance spectra from individuals with and without COVID-19, showing clear differences in electrochemical signatures. This work demonstrates the promise of nanostructured biosensors for rapid, point-of-care diagnostics in infectious disease detection and monitoring. Adapted from Ali et al., Journal of Medical Virology. 2022 Dec;94(12):5808-26.
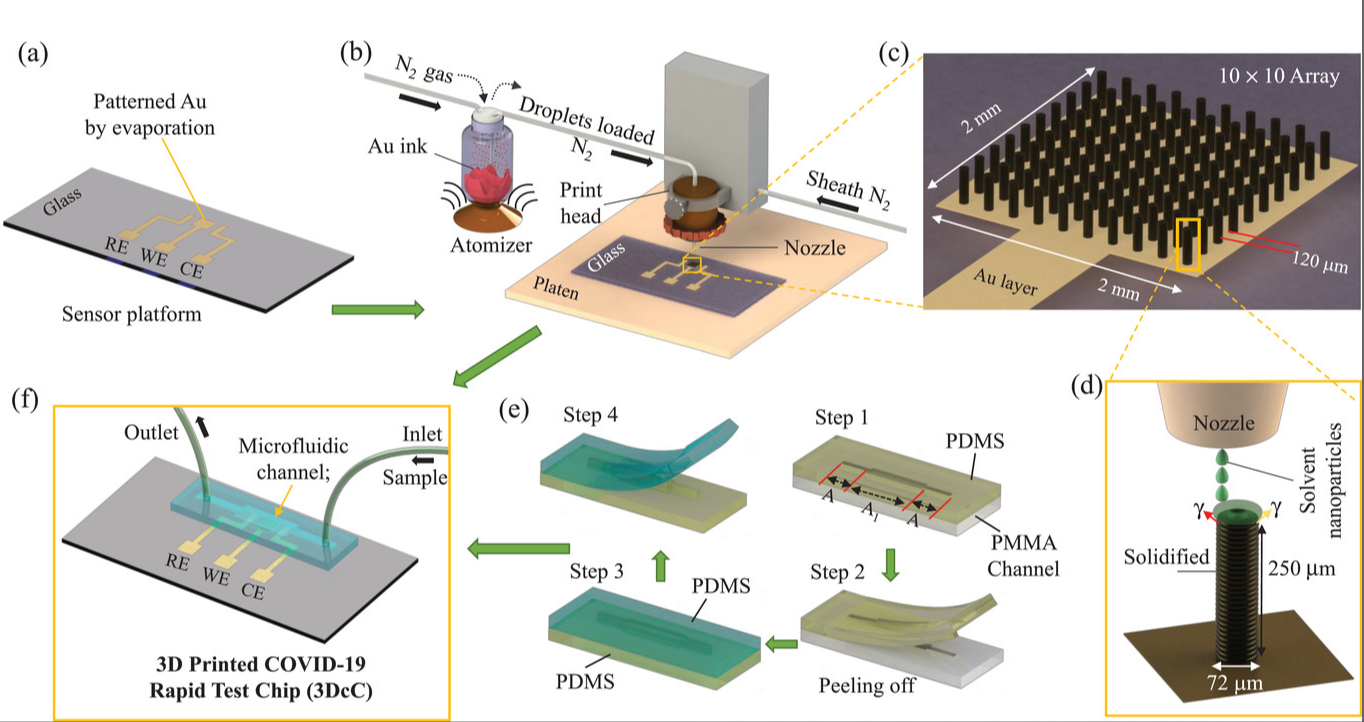
A 10-Second COVID-19 Test—A New Era in Rapid Diagnostics: Featured on the cover of Advanced Materials (Vol. 33, No. 7), this breakthrough COVID-19 antibody test delivers results in just 10 seconds. Built on aerosol jet 3D-printed gold microelectrodes coated with reduced graphene oxide and viral antigens, the biosensor enables ultra-fast, specific detection using a smartphone interface. The microfluidic chip channels the sample over functionalized pillars, where viral antibodies are instantly captured and analyzed. Compact, low-cost, and smartphone-integrated, this platform marks a game-changing advance in infectious disease diagnostics—ideal for rapid, on-site pandemic response. Adpated from Ali et al., Advanced Materials. 2021 Feb;33(7):2170046.

The cover image illustrates an innovative biosensing system for real-time soil nutrient detection. It highlights a bioengineered scaffold—graphene foam integrated with titanium nitride—designed to mimic plant root systems. This porous, microfluidic-enabled structure facilitates ion and nutrient transport, represented by red and purple markers, enabling precise biochemical sensing in agricultural environments. Showcased alongside a growing corn plant, the visual captures the synergy of materials science, microfluidics, and bioengineering for in situ soil diagnostics. This concept, developed by Liang Dong et al., advances lab-on-chip applications for sustainable farming. Adapted from Ali et al., Lab on a Chip. 2017;17(2):274-85.
Scroll for a journal articles or jump to: Book Chapters | Patents | Conference Proceedings
2025
76. Matin Ataei Kachouei, Jacob Parkulo, Samuel D. Gerrard, Tatiane Fernandes, Johan S. Osorio, & Md. Azahar Ali, Attomolar-sensitive milk fever sensor using 3D-printed multiplex sensing structures, Nature Communications, 2025, https://www.nature.com/articles/s41467-024-55535-w.

2024
75. Md. Azahar Ali and Matin Ataei Kachouei, Advancing Multi-Ion Sensing with Poly-Octylthiophene: 3D-Printed Milker-Implantable Microfluidic Device, Advanced Science, 2024, https://doi.org/10.1002/advs.202408314.

74. Shannon Chick, Matin Ataei Kachouei, Katharine Knowlton, and Md. Azahar Ali, Functionalized Graphene-Based Biosensors for Early Detection of Subclinical Ketosis in Dairy Cows, ACS Applied Materials and Interfaces, 2024, DOI: 10.1021/acsami.4c07715.

73. Md. Azahar Ali, George Fei Zhang, Chunshan Hu, Bin Yuan, Shou-Jiang Gao, and Rahul Panat, An Advanced Healthcare Sensing Platform for Direct Detection of Viral Proteins in Seconds at Femtomolar Concentrations via Aerosol Jet 3D-Printed Nano and Biomaterials, Advanced Materials Interfaces, 2024, DOI: https://doi.org/10.1002/admi.202400005.
2023
72. Kamil Reza Khondakar, Matin Ataei Kachouei, Frank Efe Erukainure, and Md. Azahar Ali, Review—Prospects in Cancer Diagnosis: Exosome-Chip for Liquid Biopsy, ECS Sensors Plus, 2023, DOI: 10.1149/2754-2726/ad08d7.
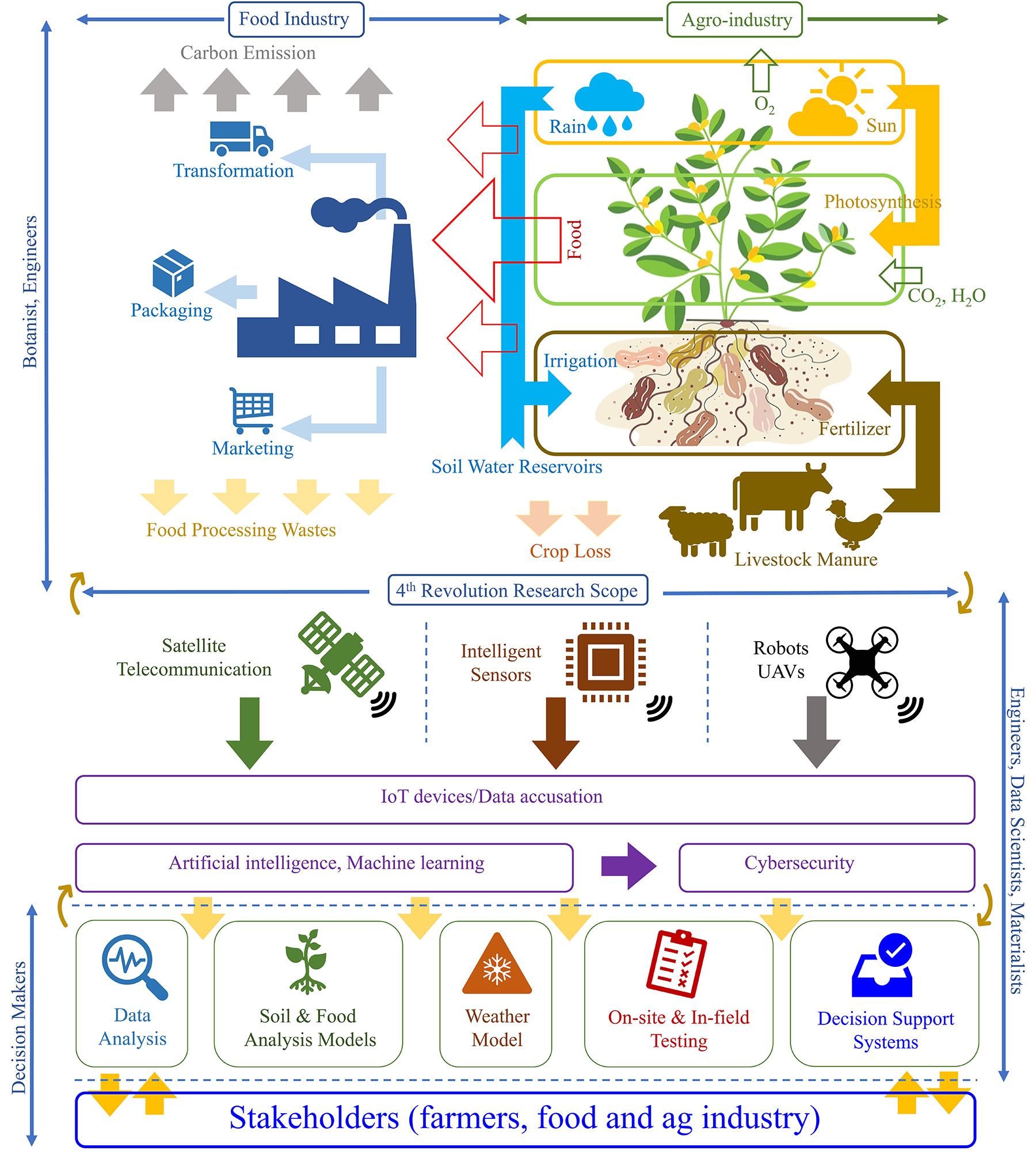
71. Matin Ataei Kachouei, Ajeet Kaushik, and Md Azahar Ali, IoT-enabled Food and Plant Sensors to Empower Sustainability, Advanced Intelligent Systems, 2023, 2300321.
2022
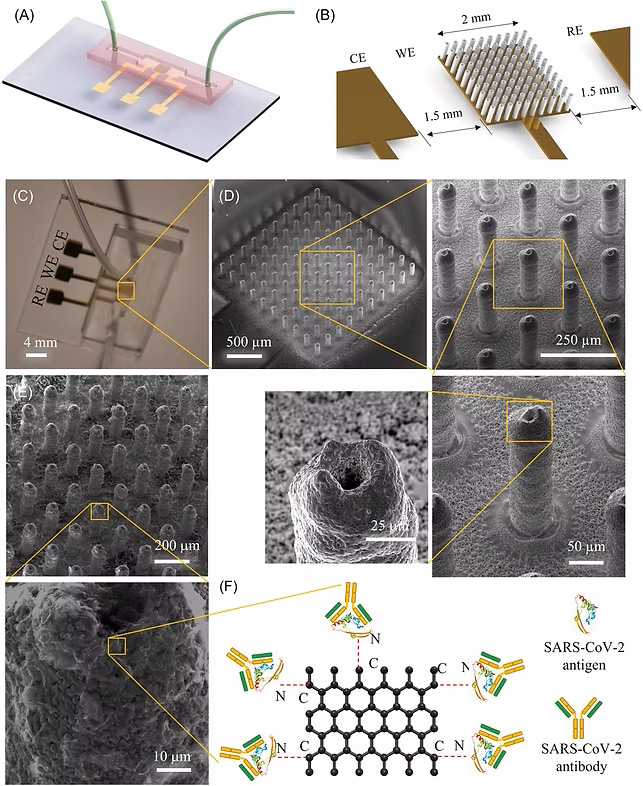
70. Md. Azahar Ali, G. Z. Fei, C. Hu, et al., Ultra-Rapid and Ultra-Sensitive Detection of SARS-CoV-2 Antibodies in COVID-19 Patients via A 3D-Printed Nanomaterial-Based Biosensing Platform, Journal of Medical Virology, 2022; doi: 10.1002/jmv.28075 (Link) (Selected as Journal cover page)
69. S. Banik, A. Uchil, T. Kalsang, S. Chakrabarty, Md. Azahar Ali, P. Srisungsitthisunti, K. K. Mahato, S. Surdo, N. Mazumder, The revolution of PDMS microfluidics in cellular biology, Crit. Rev. Biotechnol. 2022, 1-19 (doi.org/10.1080/07388551.2022.2034733) (Link).
68. Md. Azahar Ali, C. Hu, Z. Fei, B. Yuan, S. Jahan, S.-J. Gao, R. Panat, N-protein based Ultrasensitive SARS-CoV-2 Antibody Detection in Seconds via 3D Nanoprinted Microarchitected Array Electrodes, Journal of Medical Virology, 2022, 1-12 (DOI: 10.1002/jmv.27591) (Link).
2021
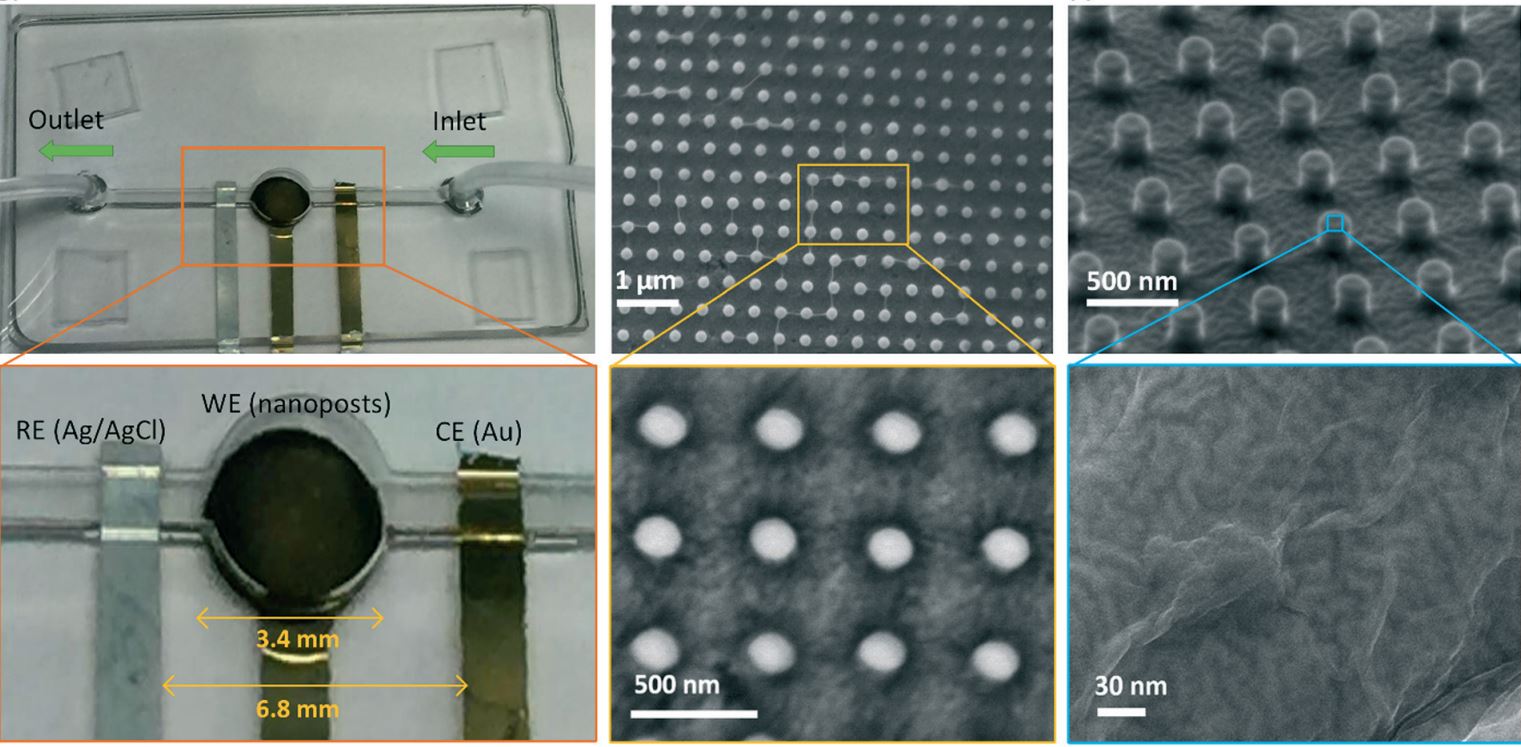
67. Md. Azahar Ali, C. Hu, B. Yuan, S. Jahan, M. S. Saleh, Z. Guo, A. J. Gellman, R. Panat, “Breaking the barrier to biomolecule limit-of-detection via 3D printed multi-length-scale graphene-coated electrodes”, Nature Communications, 2021, 12(1),7077-1 to 7077-16 (Link).
66. Md. Azahar Ali, C. Hu, E. A. Yttri, and R. Panat, “Recent Advances in 3D Printing of Biomedical Sensing Devices”, Advanced Functional Materials, 2107671, 2021 (Link), (Selected as Journal cover page)
65. Md. Azahar Ali, C. Hu, S. Jahan, B. Yuan, M. S. Saleh, E. Ju, S.-J. Gao, R. Panat, Sensing of COVID‐19 antibodies in seconds via aerosol jet nanoprinted reduced‐graphene‐oxide‐coated 3D electrodes, Advanced Materials, 2021, 33, 2006647 (Link). (Selected as Journal cover page)
64. A. Gosai, K. R. Khondakar, X. Ma, Md. Azahar Ali, Application of Functionalized Graphene Oxide Based Biosensors for Health Monitoring: Simple Graphene Derivatives to 3D Printed Platforms, Biosensors, 2021, 1(10), 384 (Link).
63. P. A. Borade, Md. Azahar Ali, S. Jahan, T. Sant, K. Bogle, R. Panat, S. M. Jejurikar, MoS2 Nanosheet-Modified NiO Layers on a Conducting Carbon Paper for Glucose Sensing, ACS Appl. Nano Mater., 2021, 4, 7, 6609–6619 (Link).
62. Y. Zhu, Y. Chen, Md. Azahar Ali, L. Dong, X. Wang, S. V. Archontoulis, J. C. Schnable, M. J. Castellano, "Continuous in situ soil nitrate sensors: the importance of high-resolution measurements across time and a comparison with salt extraction-based methods," Soil Science Society of America Journal, 2021 (DOI: 10.1002/saj2.20226) (Link).
61. A. A. Ansari, M. Alam, Md. Azahar Ali, Nanostructured CeO2:Ag platform for electrochemically sensitive detection of nitrophenol, Colloids Surf, A Physicochem Eng Asp, 2021, 126116 (Link).
2020
60. N. Singh, Md. Azahar Ali, P. Rai, I. Ghori, A. Sharma, B. D Malhotra, R. John, Dual-modality microfluidic biosensor based on nanoengineered mesoporous graphene hydrogels, Lab on a Chip, 2020, 20, 760-777 (Link).
59. Md. Azahar Ali, L. Dong, J. Dhau, A. Khosla, A. Kaushik, Perspective—electrochemical sensors for soil quality assessment, J. Electrochem. Soc, 2020, 167, 037550 (Link).
2019
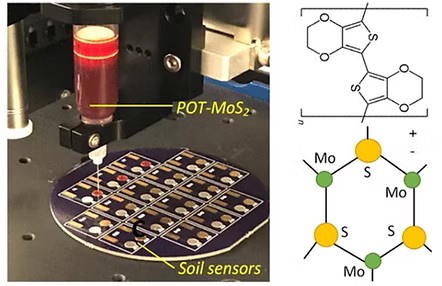
58. Md. Azahar Ali, X. Wang, Y. Chen, Y. Jiao, M. Satyanarayana, M. J. Castellano, J. C. Schnable, P. S. Schnable, and L. Dong, Continuous monitoring of soil nitrate variation using miniature sensor with poly(3-octyl-thiophene) and molybdenum disulfide nanocomposite, ACS Appl. Mater. Interfaces, 2019, 11, 29195-29206 (Link).
57. N. Singh, P. Rai, Md. Azahar Ali, R. Kumar, A. Sharma, B. D. Malhotra, R. John, Hollow-nanospheres-based microfluidic biosensors for biomonitoring of cardiac troponin I, J. Mater. Chem. B, 2019, 7, 3826-3839 (Link).
56. Md. Azahar Ali, A Special Issue on Microfluidic, Nanostructures and Biomedical Sensors, Sensor Lett. 2019, 17, 1–3 (Link).
2018
55. P. Gulati, P. Kaurb, M.V. Rajam, T. Srivastava, Md. Azahar Ali, P. Mishra, and S.S. Islam, Leukemia biomarker detection by using photoconductive response of CNT electrode: Analysis of sensing mechanism based on charge transfer induced Fermi level fluctuation, Sensors and Actuators B: Chemical, 2018, 270, 45–55 (Link).
54. Y. Wang, Md. Azahar Ali, L. Dong, and M. Lu, An optofluidic metasurface for lateral flow-through detection of cancer biomarker, Biosensors and Bioelectronics, 2018, 107, 224–229 (Link).
53. Md. Azahar Ali, S. Tabassum, Y. Wang, Q. Wang, R. Kumar, and L. Dong, Integrated dual-modality microfluidic sensor for biomarker detection, Lab on a Chip, 2018, 18, 803-817 (Link).
52. C. Singh, Md. Azahar Ali, V. Kumar, R. Ahmad, G. Sumana, Functionalized MoS2 nanosheets assembled microfluidic immunosensor for highly sensitive detection of food pathogen, Sensors and Actuators B: Chemical, 2018, 259, 1090-1098 (Link).
2017
51. N. Singh, Md. Azahar Ali, P. Rai, A. Sharma, B. D. Malhotra, and R. John, Microporous nanocomposite enabled microfluidic biochip for cardiac biomarker detection, ACS Applied Materials & Interfaces, 2017, 9, 33576−33588 (Link).
50. C. Singh, Md. Azahar Ali, V. Reddy, D. Singh, C. G.Kim, G. Sumana, and B. D. Malhotra, Biofunctionalized graphene oxide wrapped carbon nanotubes enabled microfluidic immunochip for bacterial cell detection, Sensors and Actuators B: Chemical, 2018, 255, 2495-2503 (Link).
49. Md. Azahar Ali, C. Singh, S. Srivastava, P. Admane, V. V. Agrawal, R. John, A. Pandya, L. Dong and B. D. Malhotra, Graphene oxide – metal nanocomposites for cancer biomarker detection, RSC Advances, 2017, 7, 35982–35991 (Link).
48. K. K. Reza, Md. Azahar Ali, M. K. Singh, V. V. Agrawal, and A. M. Biradar, Amperometric enzymatic determination of bisphenol A using an ITO electrode modified with reduced graphene oxide and Mn3O4 nanoparticles in a chitosan matrix, Microchimica Acta, 2017, 68, 1-8 (Link).
47. K. Mondal, Md. Azahar Ali, C. Singh, G. Sumana, B. D. Malhotra, and A. Sharma, Highly sensitive porous carbon and metal/carbon conducting nanofiber based enzymatic biosensors for triglyceride detection, Sensors and Actuators B. Chemical, 2017, 246, 202–214 (Link).
46. Md. Azahar Ali, K. Mondal, Y. Wang, N. Mahal, M. J. Castellano, A. Sharma, and L. Dong, In situ integration of graphene foam–titanium nitride based bio-scaffolds and microfluidic structures for soil nutrient sensors, Lab on a Chip, 2017, 17, 274-285 (Link). (Selected as Journal cover page)
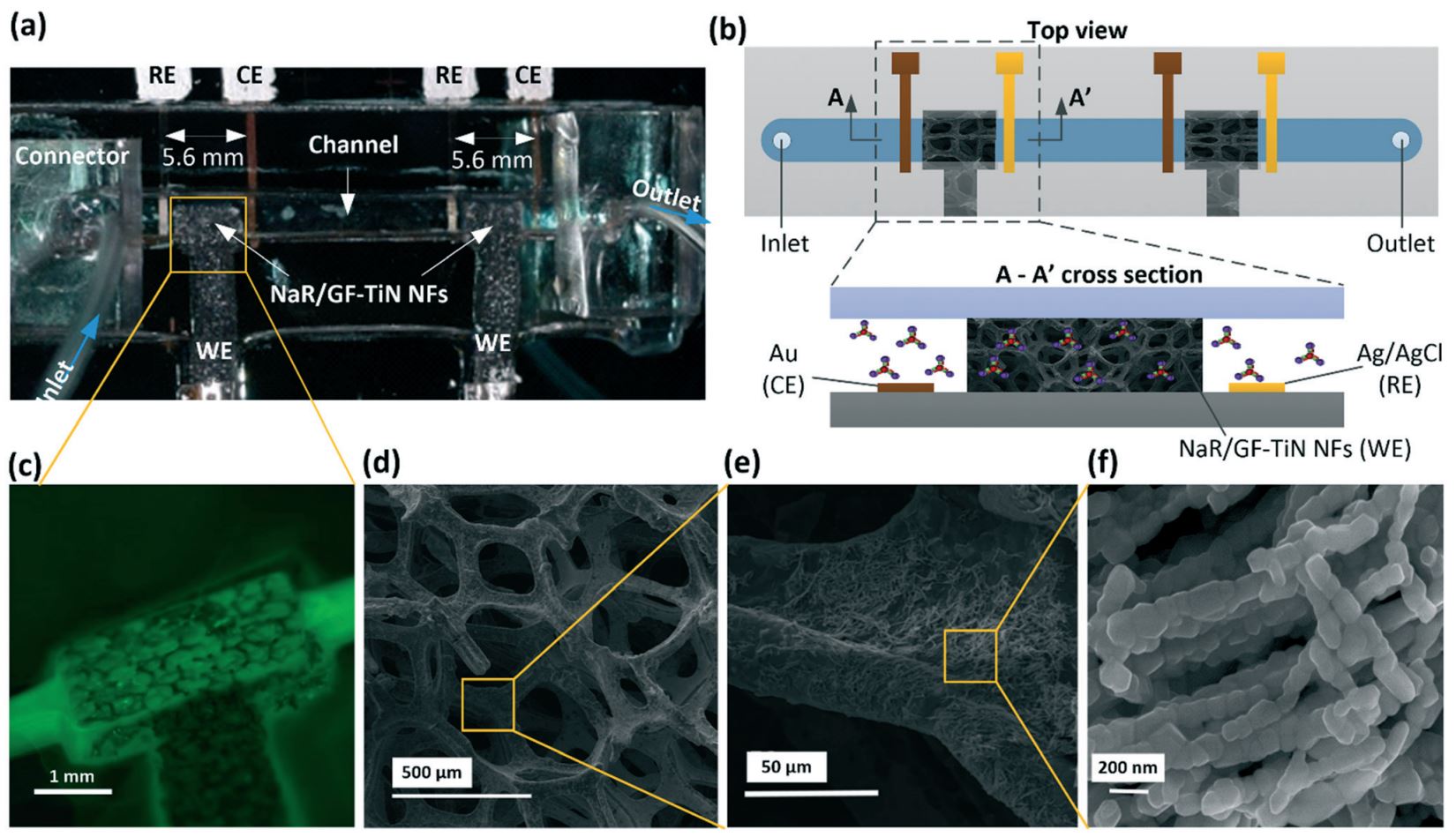
45. H. Jiang, Md. Azahar Ali, Z. Xu, L. J. Halverson, and L. Dong, Integrated microfluidic flow-through microbial fuel cells, Scientific Reports, 2017, 7: 41208. (Link).
44. Md. Azahar Ali, H. Jiang, N. Mahal, M. Castellano, and L. Dong, Microfluidic impedimetric sensor for soil nitrate detection using graphene oxide and conductive nanofibers enabled sensing interface, Sensor and Actuator B. Chemical, 239, 2017, 1289–1299 (Link).
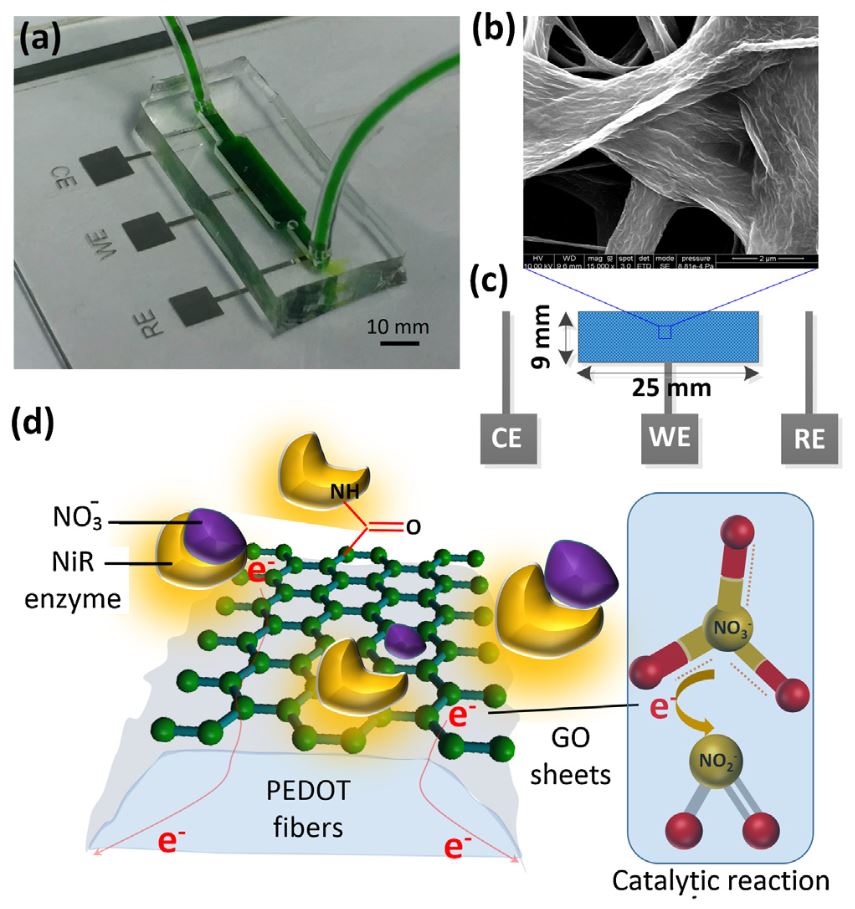
2016
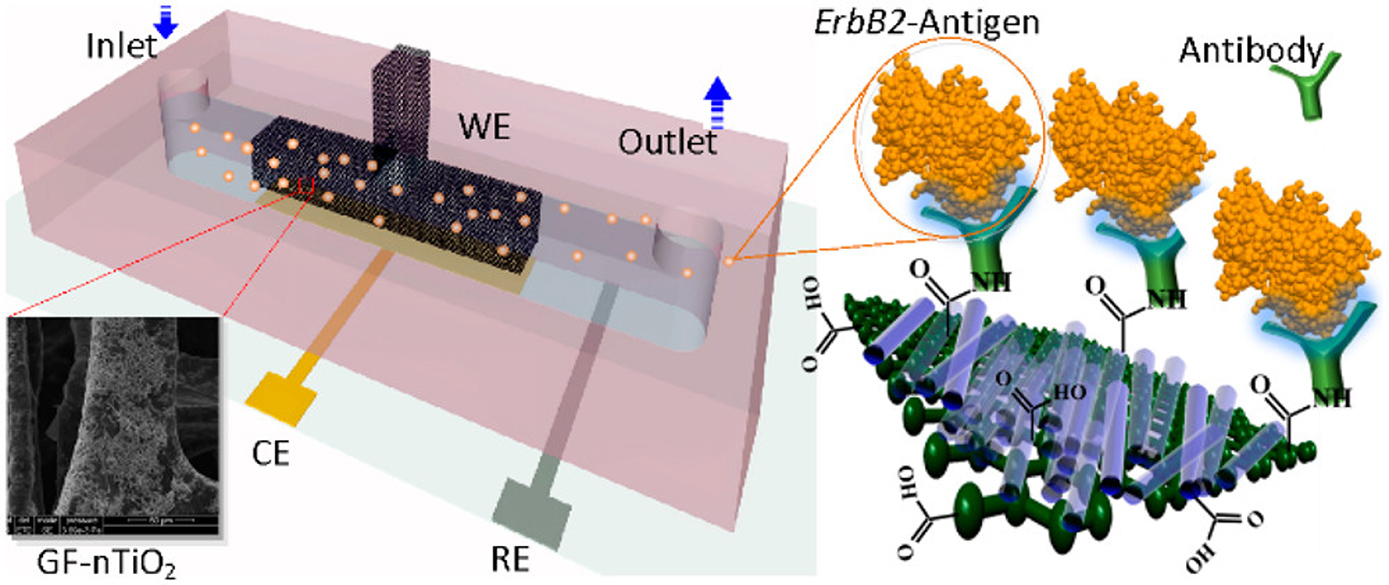
43. Md. Azahar Ali, K. Mondal, Y. Jiao, S. Oren, Z. Xua, A. Sharma, and L. Dong, Microfluidic immuno-biochip for detection of breast cancer biomarkers using hierarchical composite of porous graphene and titanium dioxide nanofibers, ACS Appl. Mater. Interfaces, 2016, 8, 20570–20582 (Link).
42. Md. Azahar Ali, W. Hong, S. Oren, Y. Wang, Q. Wang, and L. Dong, Tunable bioelectrodes with wrinkled-ridged graphene oxide surfaces for electrochemical nitrate sensors, RSC Advances, 2016, 6, 67184-67195 (Link).
41. S. Srivastava, V. Kumar, K. Arora, C. Singh, Md. Azahar Ali, N. K. Puri and B. D. Malhotra, Antibody conjugated metal nanoparticles decorated graphene sheets for mycotoxin sensor, RSC Advances, 2016, 6, 56518-56526 (Link).
40. N. Singh, Md. Azahar Ali, K. Suresh, V. V. Agrawal, P. Rai, A. Sharma, and B. D. Malhotra, and R. John, In-situ electrosynthesized nanostructured Mn3O4-polyaniline nanofibers biointerface for endocrine disrupting chemical detection, Sensor and Actuator B. Chemical, 2016, 236, 781–793 (Link).
39. Md. Azahar Ali, V. V. Agrawal, R. John, and B. D Malhotra, A biofunctionalized quantum dot–nickel oxide nanorod based smart platform for lipid detection, Journal of Material Chemistry B, 2016, 4, 2706-2714 (Link).
38. Md. Azahar Ali, C. Singh, K. Mondal, S. Srivastava, A. Sharma and B. D Malhotra, Mesoporous few-layer graphene platform for affinity biosensing application, ACS Appl. Mater. Interfaces, 2016, 8, 7646–7656 (Link).
37. C. Singh, Md. Azahar Ali, and G. Sumana, Green synthesis of graphene based biomaterial using fenugreek seeds for lipid detection, ACS Sustainable Chem. Eng., 2016, 4, 871–880 (Link).
36. K. Mondal, Md. Azahar Ali, S. Srivastava, B. D Malhotra and A. Sharma, A novel lithography free microfabrication of micro/nano channels embedded in porous carbon electrode for biosensing application, Sensor and Actuator B Chemical 229, 2016 82–91 (Link).
2015
35. M. K. Patel, Md. Azahar Ali, S. Krishnan, V. V. Agrawal, A. A. Al Kheraif, H. Fouad, Z. Ansari, S. G. Ansari and B. D. Malhotra, A label-free photoluminescence genosensor using nanostructured magnesium oxide, Scientific Reports, 2015, 5, 17384 (Link).
34. K. K. Reza, Md. Azahar Ali, S. Srivastava, V. V. Agrawal and A.M. Biradar, Tyrosinase conjugated reduced graphene oxide based biointerface for bisphenol A sensor. Biosensors and Bioelectronics, 2015, 74, 644–651 (Link).
33. P. R. Solanki, M. K. Patel, Md. Azahar Ali, B. D. Malhotra, Chitosan modified nickel oxide platform for biosensing applications, Journal of Material Chemistry B, 2015, 3, 6698-6708 (Link).
32. H. Dhyani, Md. Azahar Ali, S. P. Pal, S. Srivastava, P. R. Solanki, B. D. Malhotra and P. Sen, Mediator-free biosensor using chitosan capped CdS quantum dots for detection of total cholesterol, RSC Advances, 2015, 5, 45928-45934 (Link).
31. A. S. Ghrera, C. M. Pandey, Md. Azahar Ali, B. D. Malhotra, Quantum dot‒based microfluidic biosensor for cancer detection, Applied Physics Letters, 2015, 106, 193703 (Link).
30. Md. Azahar Ali, K. Mondal, C. Singh, B. D Malhotra, and A. Sharma, Anti-epidermal growth factor receptor conjugated mesoporous zinc oxide nanofibers for breast cancer diagnostics, Nanoscale, 2015, 7, 7234-7245, (Link).
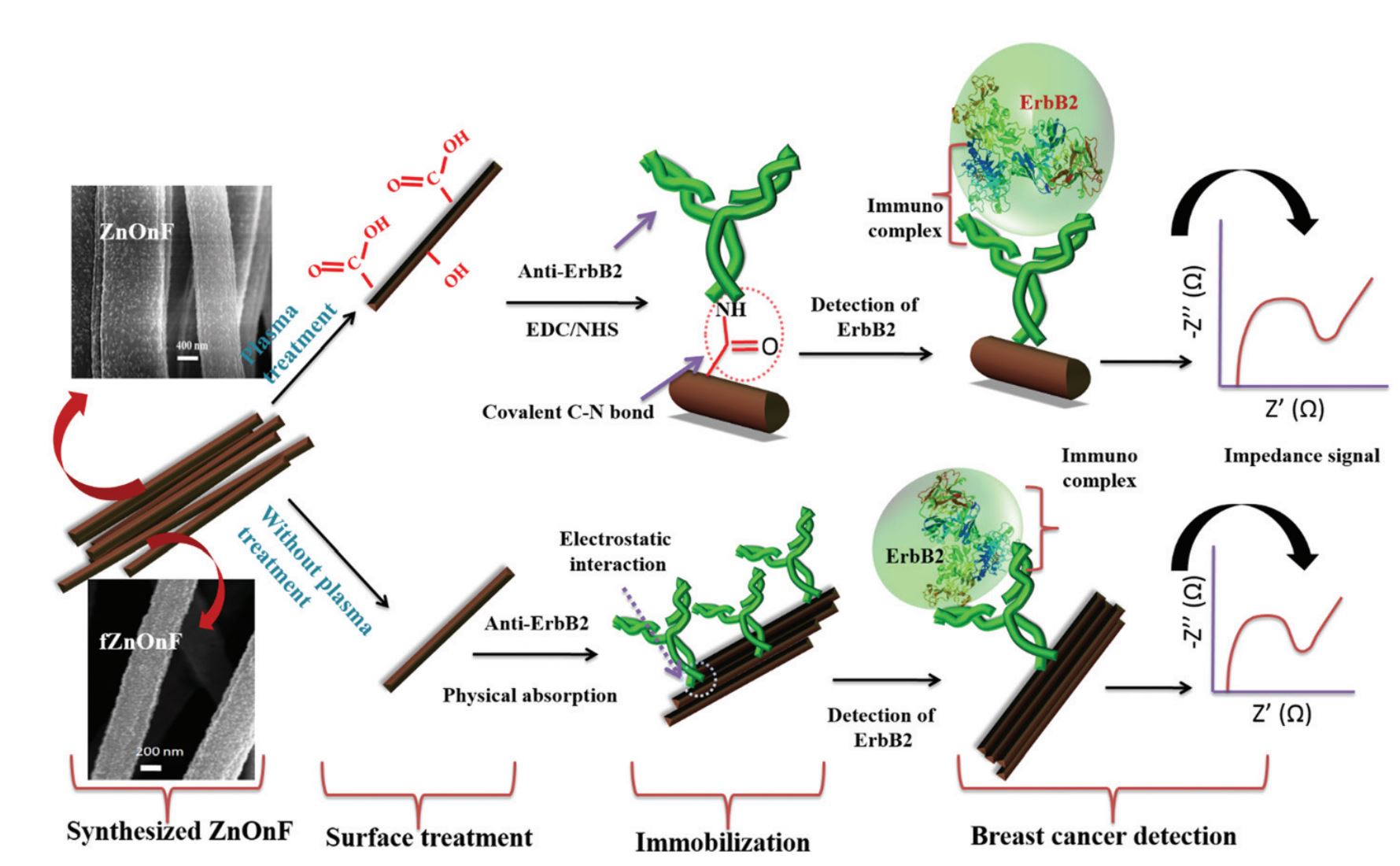
29. Md. Azahar Ali, P. R. Solanki, S. Srivastava, S. Singh, V. V. Agrawal, R. John, and B. D Malhotra, Protein functionalized carbon nanotubes based smart lab-on-a-chip, ACS Appl. Mater. Interfaces, 2015, 7, 5837-46 (Link).
28. N. Singh, K. K. Reza, Md. Azahar Ali, V. V. Agrawal, A. M. Biradar, Self-assembled nanostructured rutile TiO2 platform for estrogenic substance detection. Biosensensors and Bioelectronics, 2015, 22, 633-641 (Link).
27. Md. Azahar Ali, S. Srivastava, K. Mondol, V. V. Agrawal, R. John, A. Sharma and B. D. Malhotra, A surface functionalized nanoporous integrated microfluidic biochip, Nanoscale, 2014, 6, 13958-13969 (Link).
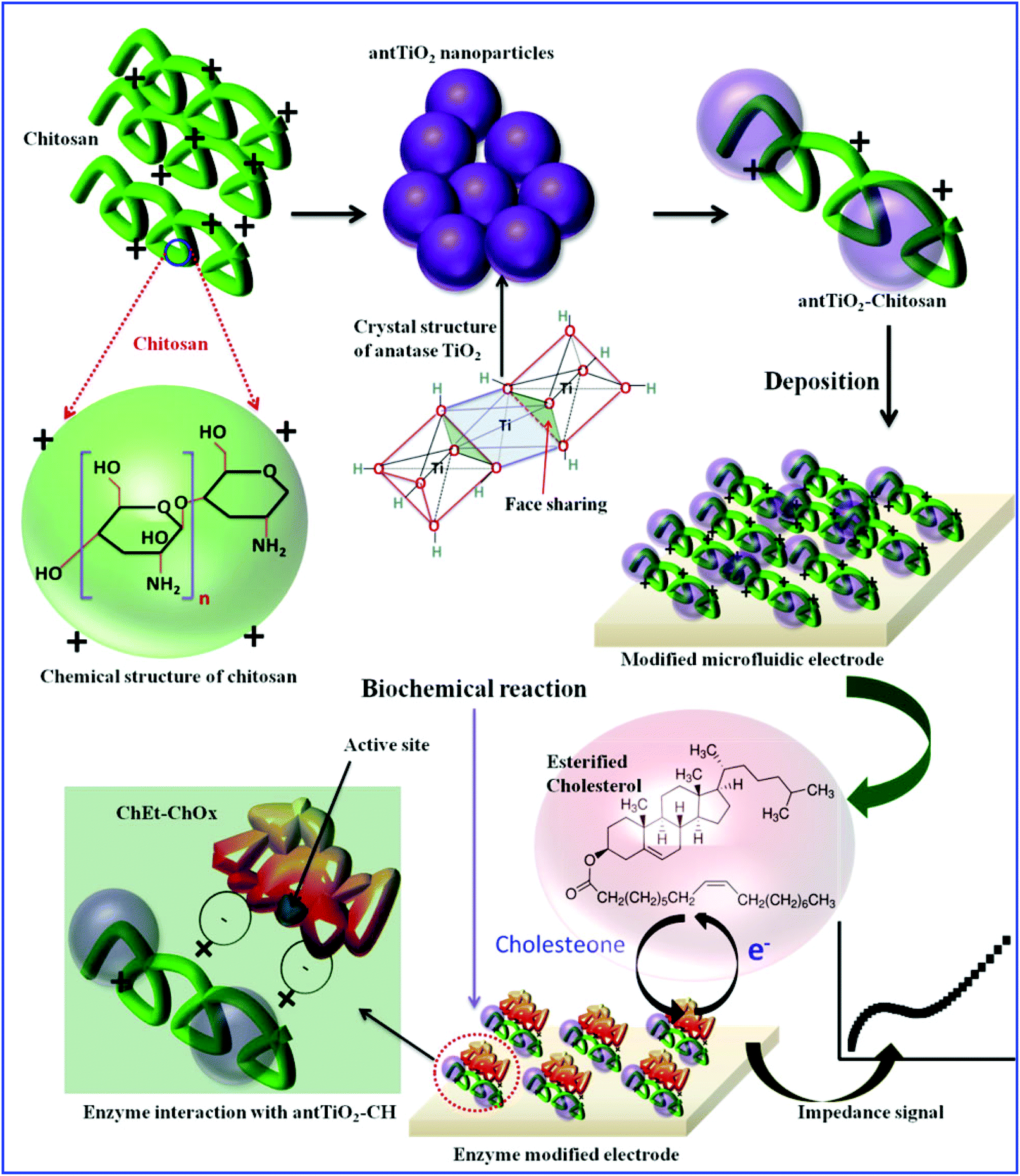
26. S. Srivastava, S. Abraham, C. Singh, Md. Azahar Ali, A. Srivastava, G. Sumana, and B. D. Malhotra, Protein conjugated carboxylated gold@reduced graphene oxide for aflatoxin B1 detection, RSC Advances, 2015, 5, 5406-5414 (Link).
2014
25. P. R. Solanki, S. Srivastava, Md. Azahar Ali, R. K. Srivastava, G. Sumana, A. Srivastava, and B. D. Malhotra, Protein conjugated reduced graphene oxide-titania platform for label-free biosensor application, RSC Advances, 2014, 4, 60386-60396 (Link).
24. R. Sharma, Md. Azahar Ali, N. Selvi, V. Singh, R. Sinha, and Ved V. Agrawal, Electrochemically assembled gold nanostructures platform: electrochemistry, kinetic analysis and biomedical application, Journal Physical Chemistry C, 2014, 118, 6261–6271 (Link).
23. K. Mondal, Md. Azahar Ali, V. V. Agrawal, B. D. Malhotra, and A. Sharma, Highly sensitive biofunctionalized mesoporous nanofibers based interface for biomedical application, ACS Appl. Mater. Interfaces, 2014, 6, 2516−2527 (Link).
22. Md. Azahar Ali, S. Srivastava, M. K. Pandey, V. V. Agrawal, R. John, and B. D Malhotra, Protein–conjugated quantum dots interface: binding kinetics and label-free lipid detection, Analytical Chemistry, 2014, 86, 1710–1718 (Link).
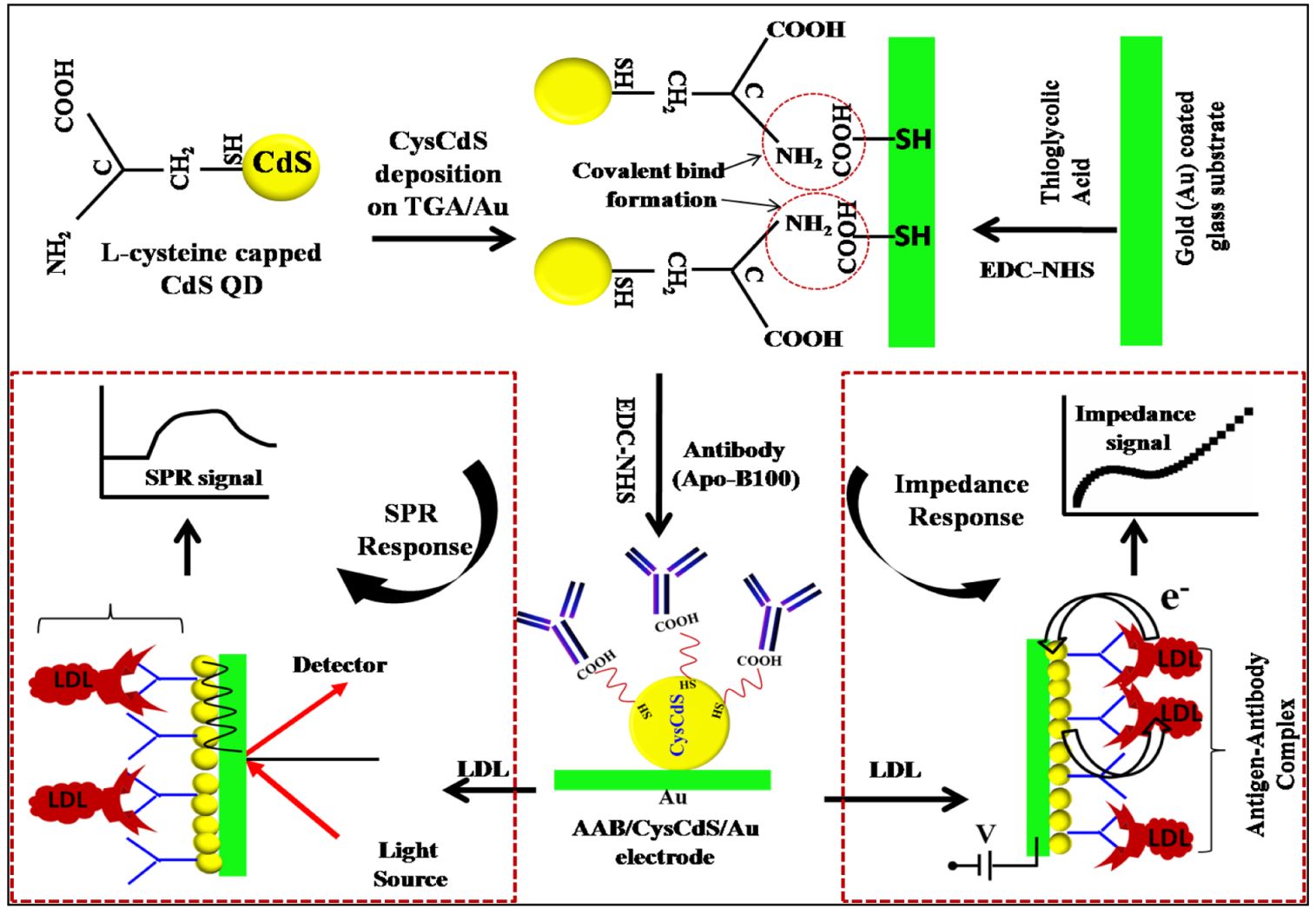
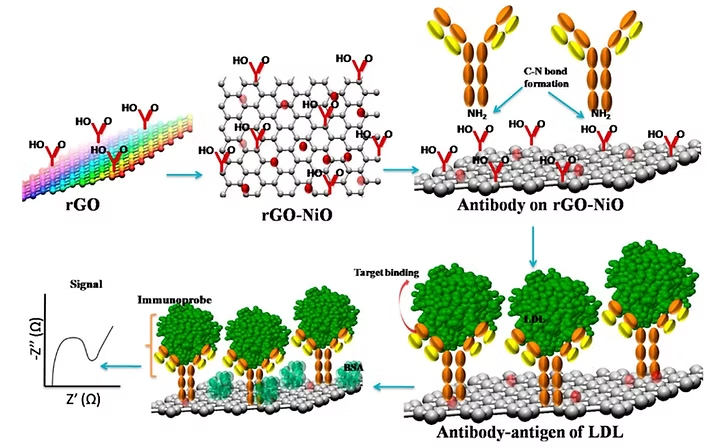
21. Md. Azahar Ali, K. Kamil Reza, S. Srivastava, M. K. Pandey V. V. Agrawal, R. John, B. D Malhotra, Functionalized reduced graphene oxide based interface for label-free low-density lipoprotein detection, Langmuir, 2014, 30, 4192–4201 (Link).
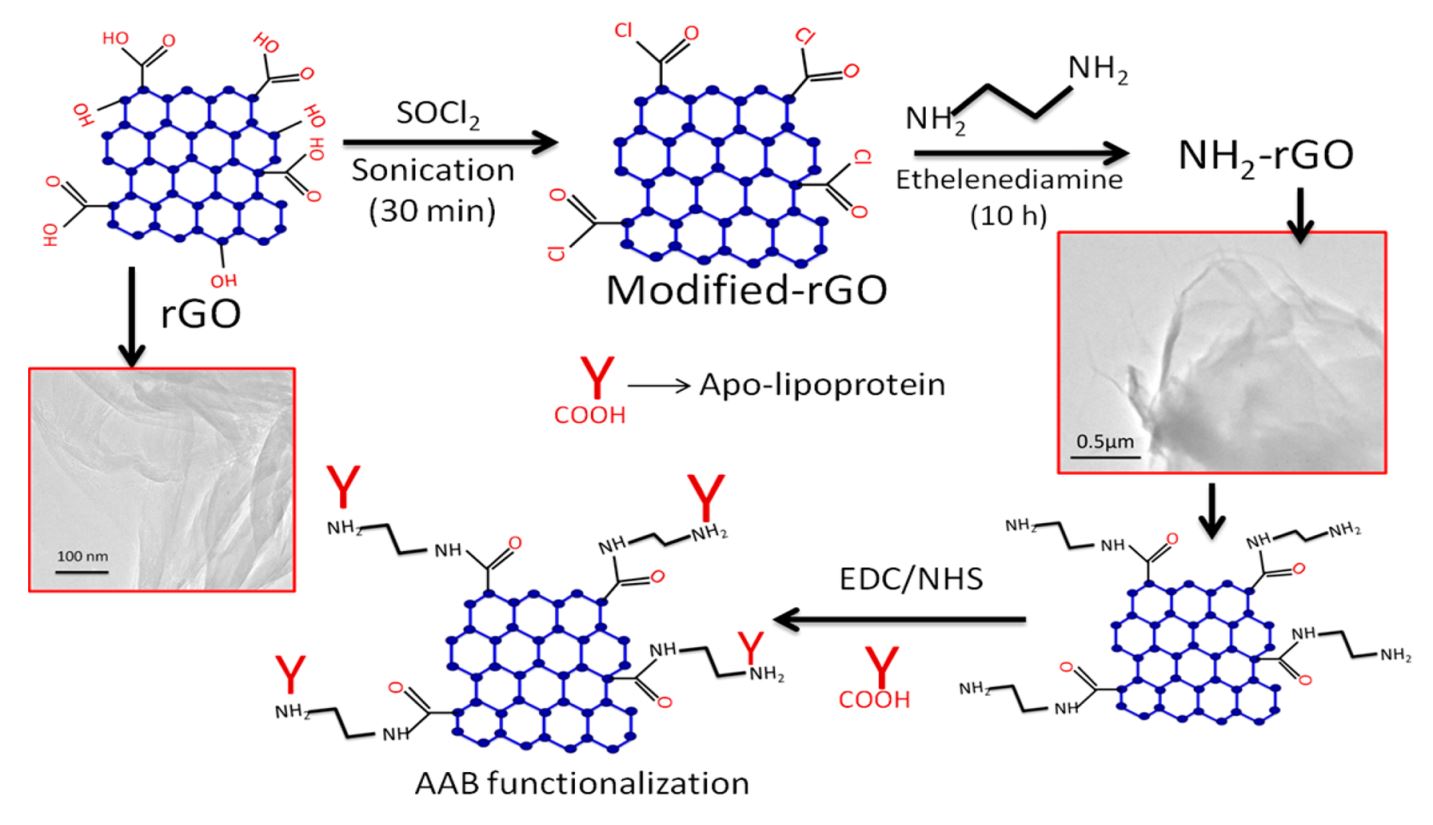
20. Md. Azahar Ali, N. Singh, S. Srivastava, V. V. Agrawal, R. John, B. D. Malhotra, Chitosan-modified carbon nanotubes-based platform for low-density lipoprotein detection, Applied Biochemistry and Biotechnology, 2014, 74, 926-35 (Link).
19. S. Srivastava, Md. Azahar Ali, S. Omrao, U. Parashar, A. Srivastava, G. Sumana, S. S. Pandey, S. Hayase, B. D. Malhotra, Graphene oxide-based biosensor for food toxin detection, Applied Biochemistry and Biotechnology, 2014, 174, 960-970 (Link).
18. B. D. Malhotra, S. Srivastava, Md. Azahar Ali, C. Singh, Carbon nanomaterials based biosensors for food toxins detection, Applied Biochemistry and Biotechnology, 2014, 174, 880-896 (Link).
2013
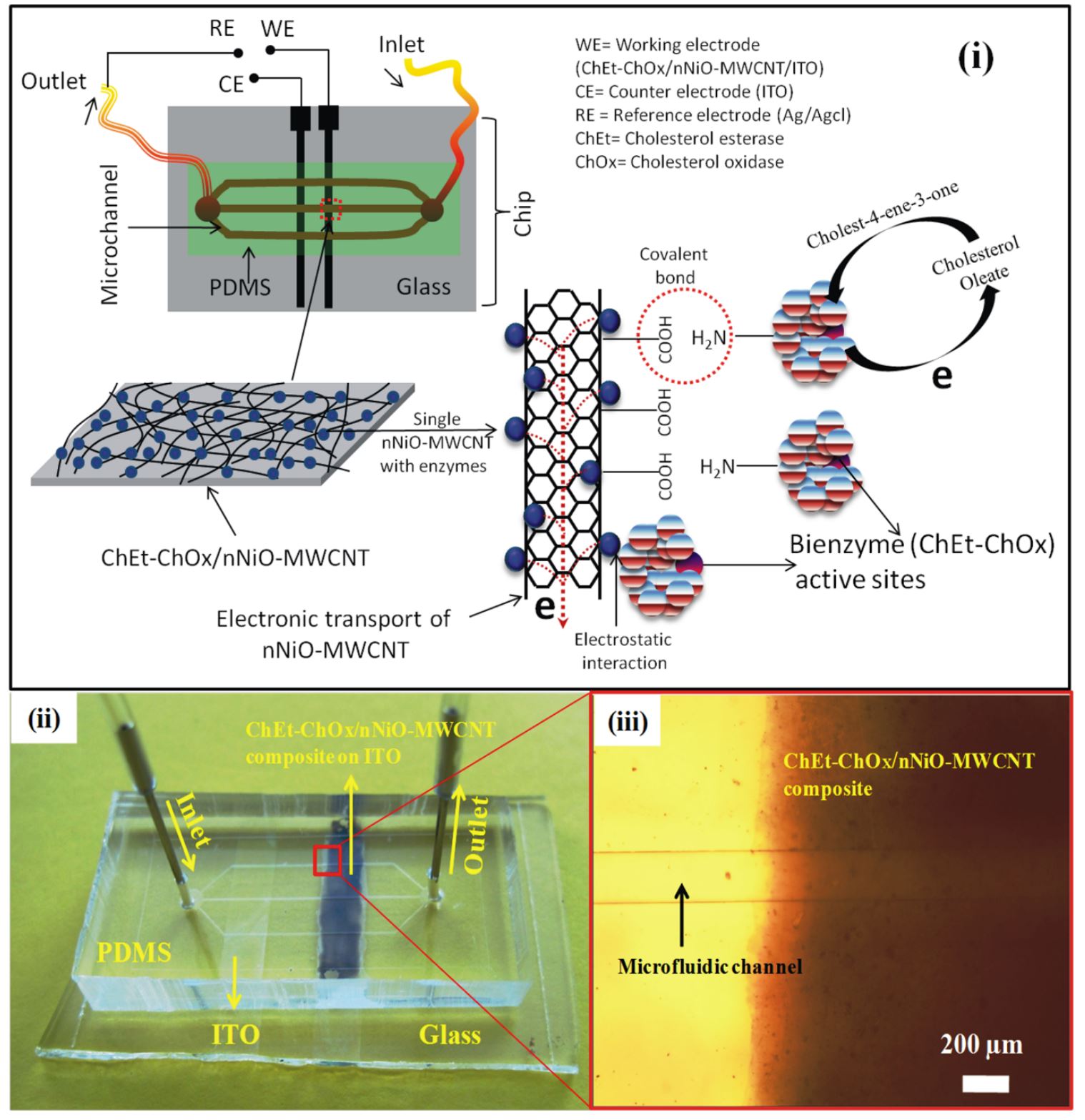
17. Md. Azahar Ali, S. Srivastava, P. R. Solanki, V. Reddy, V. V. Agrawal, C. Kim, R. John, and B. D. Malhotra, Highly efficient bienzyme functionalized nanocomposite-based microfluidics biosensor platform for biomedical application, Scientific Reports, 2013, 3, 2661, 1-9 (Link).
16. P. R. Solanki, Md. Azahar Ali, A. Kaushik, and B. D. Malhotra, Label-free capacitive immunosensor based on nanostructured cerium oxide, Advanced Electrochemistry, 2013, 1(2), 92-97 (Link).
15. P. R. Solnaki, Md. Azahar Ali, V. V. Agrawal, A. K. Srivastava, R. K. Kotnala, B. D. Malhotra, Highly sensitive biofunctionalized nickel oxide nanowires based immunosensor for cholera detection, RSC Advances, 2013, 3, 16060 (Link).
14. M. K. Patel, Md. Azahar Ali, S. Srivastava, V. V. Agrawal, S.G. Ansari, and B. D. Malhotra, Magnesium oxide grafted carbon nanotubes based impedimetric genosensor for biomedical application, Biosensors and Bioelectronics, 2013, 50, 406–413 (Link).
13. S. Dev$, S. Kumar$, Md. Azahar Ali$, P. Anand, R. John, V. V. Agrawal, R. John, and B. D. Malhotra, Microfluidics-integrated biosensors: prospects for point-of-care diagnostics, Biotechnology Journal, 2013, 8, 1267-79 (Link; $-equal contribution).
12. C. Singh, S. Srivastava, Md. Azahar Ali, T. K. Gupta, G. Sumana, A. Srivastava, R. B. Mathur, and B. D. Malhotra, Carboxylated multiwalled carbon nanotubes based biosensor for aflatoxin detection, Sensors and Actuators B. Chemical, 2013, 185, 258–264 (Link).
11. M. K. Patel, Md. Azahar Ali, V. V. Agrawal, Z. A, Ansari, B. D. Malhotra, and S. G. Ansari, Nanostructured magnesium oxide biosensing platform for cholera detection, Applied Physics Letters, 2013, 102, 144106 (Link).
10. A. C. Roy, Nisha V. S., C. Dhand, Md. Azahar Ali, and B. D. Malhotra, Molecularly imprinted polyaniline-polyvinyl sulphonic acid composite based sensor for para-nitrophenol detection, Analytical Chimica Acta, 2013, 777, 63–71 (Link).
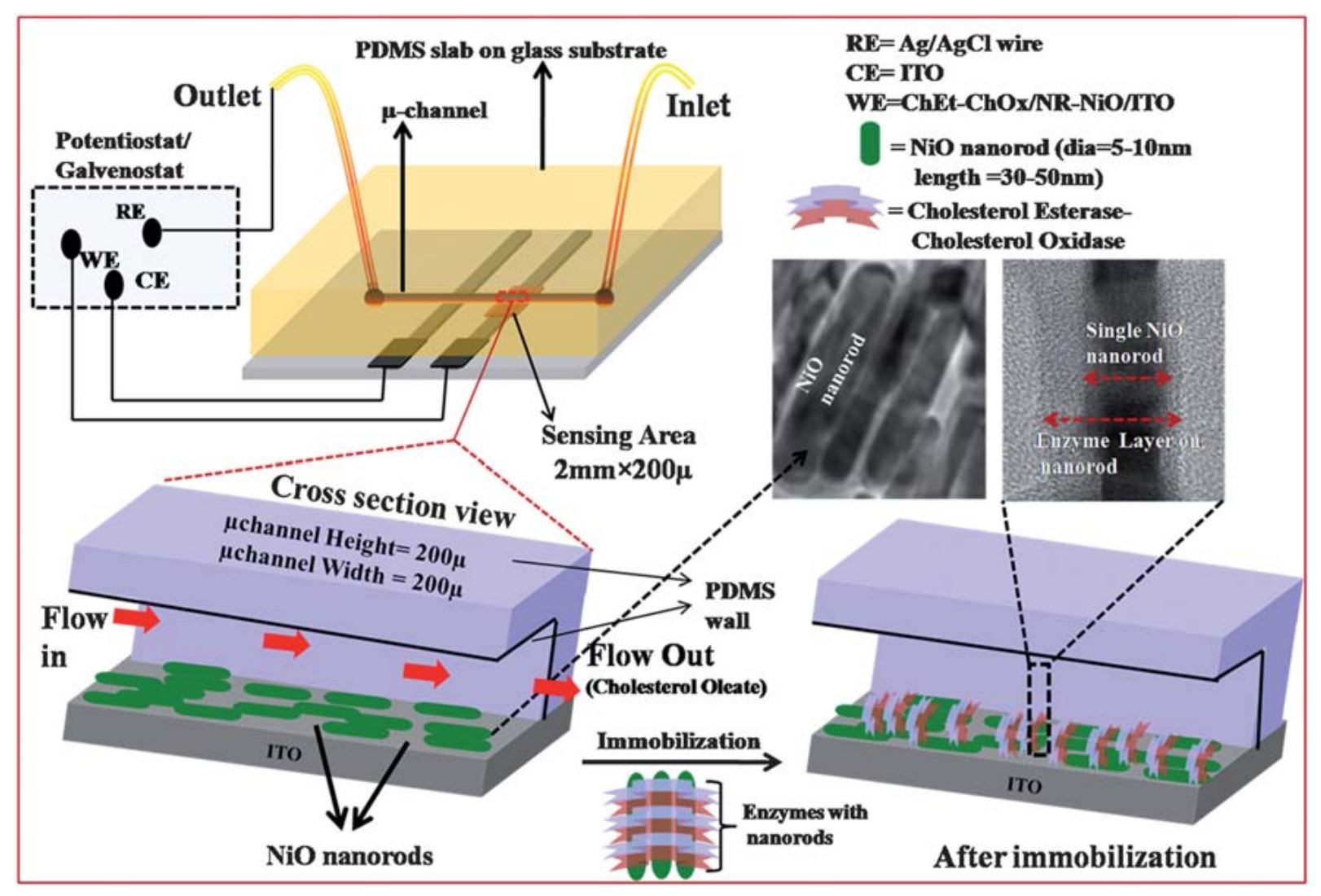
9. Md. Azahar Ali, P. R. Solanki, M.K. Patel, H. Dhayani, V.V. Agrawal, R. John, and B. D. Malhotra, A highly efficient microfluidic nano biochip based on nanostructured nickel oxide, Nanoscale, 2013, 5, 2883-2891 (Link).
8. M. K. Patel$, Md. Azahar Ali$, Md. Zafaryab, V. V. Agrawal, M. M. A. Rizvi, Z. A. Ansari, S. G. Ansari, B. D. Malhotra, Biocompatible nanostructured magnesium oxide-chitosan platform for genosensing applications, Biosensors and Bioelectronics, 2013, 45, 181–188 (Link; $-equal contribution).
7. S. Srivastava, V. Kumar, Md. Azahar Ali, P. R. Solanki, A. Srivastava, G. Sumana, P. S. Saxena, and B. D. Malhotra, Electrophoretically deposited reduced graphene oxide platform for food toxin detection, Nanoscale, 2013, 5, 3043 (Link).
6. S. Srivastava, Md. Azahar Ali, P.R. Solanki, P.M. Chavhan, M. K. Pandey, A. Mulchandani, A. Srivastava, and B. D. Malhotra, Mediator-free microfluidics biosensor based on titania–zirconia nanocomposite for urea detection, RSC Advances, 2013, 3, 228-235 (Link).
2012
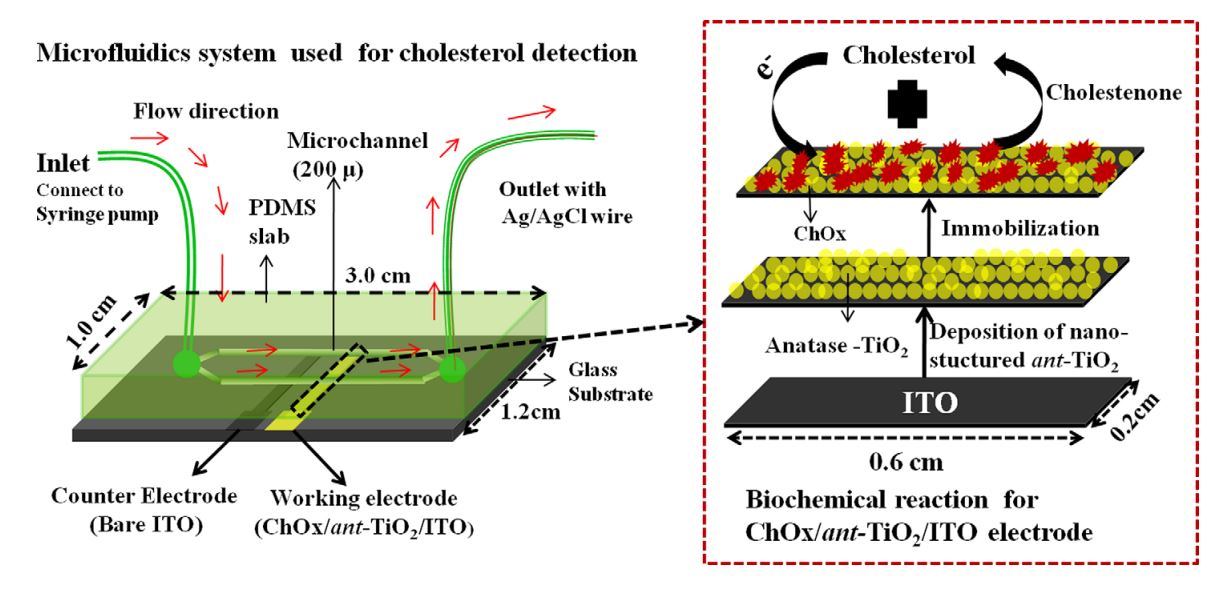
5. Md. Azahar Ali, S. Srivastava, P. R. Solanki, V V Agrawal, R. John, B. D. Malhotra, Nanostructured anatase-titanium dioxide based platform for application to microfluidics cholesterol biosensor, Appl. Phys. Lett., 2012, 101, 084105 (Link).
4. H. Dhyani, Md. Azahar Ali, M. K. Pandey, B.D. Malhotra and P. Sen, Electrophoretically deposited CdS quantum dots based electrode for biosensor application, Journal of Material Chemistry, 2012, 22, 4970 (Link).
2011
3. S. Srivastava, P. R. Solanki, A. Kaushik, Md. Azahar Ali, A. Srivastava, and B. D. Malhotra, A self-assembled monolayer based microfluidic sensor for urea detection, Nanoscale, 2011, 3, 2971-7 (Link).
2. Abdul Barik, P R. Solanki, A. Kaushik, Md. Azahar Ali, M. K. Pandey, and B. D. Malhotra, Ponyaniline- carboxymethayl cellulose nanocomposite for cholesterol detection, Journal of Nanoscience Nanotechnology, 2010, 10, 6479-88 (Link).
1. Md. Azahar Ali, A. Ansari, A. Kaushik, P. Solanki, A. Barek, and B. D. Malhotra, Nanostructured zinc oxide film for urea sensor, Materials Letters, 2009, 63, 2473–2475 (Link).
Book Chapters
1. A. Gosai and Md. Azahar Ali* “Metal oxides based microfluidic biosensing (Chapter, pages: 233-258); Metal Oxides for Biomedical and Biosensor Applications,” 1st Edition, Elsevier, 2021, ISBN: 9780128230589 (Link).
2. Md. Azahar Ali and Bansi D. Malhotra, “Nanomaterials for Biosensors: Fundamentals and Applications, (Micro and Nano Technologies) 1st Edition, Elsevier, 2017, Hardcover ISBN: 9780323449236, eBook ISBN: 9780128135150 (Link).
3. Md. Azahar Ali and Chandan Singh, “Enzymatic Biosensors (Chapter); Nanobiotechnology for Sensing Applications: From Lab to Field” pp: 161-182, CRC Press, a Tylor and Francis Group, 2016, New Jersey, USA, ISBN: 9781771883283, E-Book ISBN: 9781771883290 (Link).
Top of Page [↑]
Patents
1. Md. Azahar Ali, Chunsahn Hu, Bin Yuan, M. Sadeq. Saleh, Rahul Panat, Rapid Detection of Pathogens using Three-Dimensional Architectures as Electrochemical Sensing Elements, International Patent Number WO 2022 006520, published January 6, 2022 (Link).
2. Md. Azahar Ali, L. Dong, Y. Jiao, Y. Chen, U.S. Patent No. 11,378,541, Filed Self-contained, automated, long-term sensor system for monitoring of soil nutrients in fields (Link).
3. Md. Azahar Ali, S. Tabassum, Q. Wang, R. Kumar, L. Dong, Integrated dual-modality microfluidic sensor for biomarker detection, 2021, U.S. Patent US11022610B1 (Link).
4. Md. Azahar Ali, L. Dong, X. Wang, M. Castellano, Miniature sensors with probe insertable into and for obtaining measurements from plants and a variety of other mediums, U.S. Patent 10,921,303 (Link)
5. Md. Azahar Ali, A. Sharma, K. Mondal, B. D Malhotra, C. Singh, G. Sumana, Silver nanoparticles impregnated nanoporous carbon nanofibers platform for biosensor application, 2016, Indian Patent Office, Filling application date: 04/08/2016, Application No: 201611026698, Published on 09-02-2018, Official Journal No. 06/2018.
Top of Page [↑]
Conference Proceedings
1. Md. Azahar Ali, X. Wang, Y. Jiao, Y. Chen, L. Dong, Novel all-solid-state soil nutrient sensor using nanocomposite of poly(3-octyl-thiophene) and molybdenum sulfate, Transducers 2019 - EUROSENSORS XXXIII Berlin, GERMANY, 23-27 June 2019, 170-173 (Link).
2. X. Wang, Y. Tian, Y. Chen, Y. Jiao, Md. Azahar Ali, L. Wei, and L. Dong, Low-cost extended-gate field effect transistor sensing system based on a channel structured reference electrode, Transducers 2019 - EUROSENSORS XXXIII Berlin, GERMANY, 23-27, 2019, 1383-1384 (Link).
3. Md. Azahar Ali, S. Tabassum, Q. Wang, Y. Wang, R. Kumar and L. Dong, Plasmonic-electrochemical dual modality microfluidic sensor for cancer biomarker detection, In Micro Electro Mechanical Systems (MEMS), 2017 IEEE 30th International Conference on 2017 Jan 22, pp. 390-393 (Link).
4. H. Jiang, Md. Azahar Ali, Y. Jiao, B. Yang, and L. Dong, In-situ, real-time monitoring of nutrient uptake on plant chip integrated with nutrient sensor, In Solid-State Sensors, Actuators and Microsystems (Transducers 2017), The 19th International Conference on June 18-22, 2017, pp 289-292 (Link).
5. Md. Azahar Ali, K. Mondal, Y. Wang, N. Mahal, M. J. Castellano, A. Sharma and L. Dong, Microfluidic detection of soil nitrate ions using novel electrochemical foam electrode, In Micro Electro Mechanical Systems (MEMS), 2017 IEEE 30th International Conference on 2017, pp. 482-485 (Link).
6. Md. Azahar Ali, S. Oren, Y. Jiao, Y. Wang, Z. Xu, and L. Dong, Microfluidic label-free immunochip for early diagnostics of breast cancer using functionalized porous graphene, Technical Digest: Solid-State Sensors, Actuators, and Microsystems Workshop 2016 Hilton Head, SC, USA. 05-09 JUN 2016, pp: 284-285.
7. S. Oren, S. Tabassum, Y. Jiao, Md. Azahar Ali, and L. Dong, Wearable graphene sensors on adhesive tapes, Technical Digest: Technical Digest: Solid-State Sensors, Actuators, and Microsystems Workshop 2016 Hilton Head, SC, USA. 05-09 JUN 2016, pp: 110-111.
8. H. Jiang, Md. Azahar Ali, Z. Xu, L. J. Halverson, and L. Dong, Microfluidic Flow-Through Microbial Fuel Cell, Hilton Technical Digest: Solid-State Sensors, Actuators, and Microsystems Workshop 2016 Hilton Head, SC, USA. 05-09 JUN 2016, pp: 384-387.
9. S. Tabassum, Q. Wang, W. Wang, S. Oren, Md. Azahar Ali, R. Kumar, and L. Dong, Plasmonic crystal gas sensor incorporating graphene oxide for detection of volatile organic compounds. In Micro Electro Mechanical Systems (MEMS), 2016 IEEE 29th International Conference on 2016 Jan 24, pp. 913-916 (Link).
10. A. A. Ansari, Md. Azahar Ali and B. D. Malhotra, Electrochemical urea biosensor based on sol-gel derived nanostructured cerium oxide, Journal of Physics: Conference Series, 2012, 358, 012006 (Link).
11. H. Dhyani, S. Srivastava, Md. Azahar Ali, B. D. Malhotra and P. Sen, Fabrication of nanocrystalline CdS electrode via chemical bath deposition technique for application to cholesterol sensor, Journal of Physics: Conference Series, 2012, 358, 012008 (Link).







